Interconversion enzymes in androgenetic alopecia
Research studies in patients with androgen insensitivity syndromes and 5alpha – reductase type-2 deficiencies indicate that androgenetic alopecia is induced by activation of follicular androgen receptors by dihydrotestosterone (DHT), a potent stimulator of hair loss in the scalp. Although the level of DHT may be directly dependent on the activity of 5 -reductase, it is also obviously affected by both the supply of androgen precursors and the metabolism of DHT.
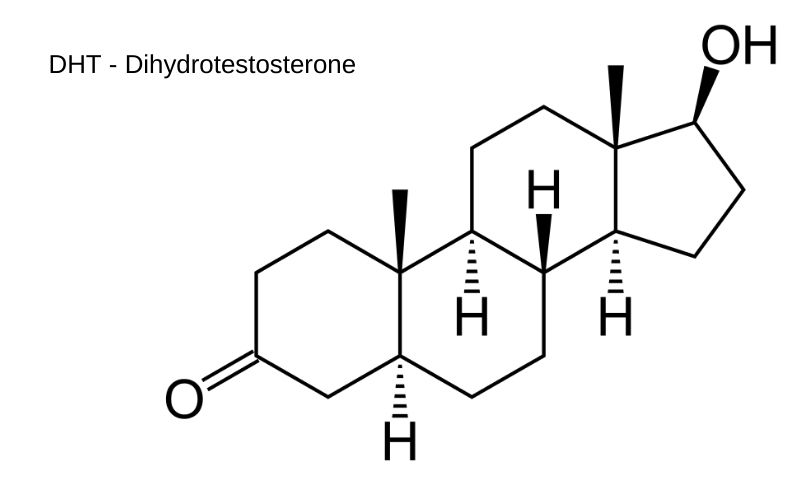
Androgen synthesis begins with cholesterol, which is converted to pregnenolone. Recent work has shown that on the scalp there are local differences in the amounts of steroid metabolizing enzymes that convert weak androgens to more potent androgens. This is important because the skin is an endocrine target tissue for androgen hormone action, similar to the ovaries, testes, and adrenal gland. It is known that weak and abundant precursor hormones such as dehydroepiandrosterone can metabolize to more potent androgens such as testosterone and dihydrotestosterone (DHT).
The enzymes that are responsible for synthesis of androgens are localized in the sebaceous glands and hair follicles of scalp skin. The skin is an active site of androgen metabolism where testosterone, androstenedione, and dehydro-epiandrosterone (DHEA) are metabolized extensively. That is because the skin has the potential to mediate androgen action without relying on elevated systemic levels of testosterone or DHT. Testosterone is the major precursor of DHT in men, but other weaker hormones, such as DHEA are the major precursors of DHT in women.
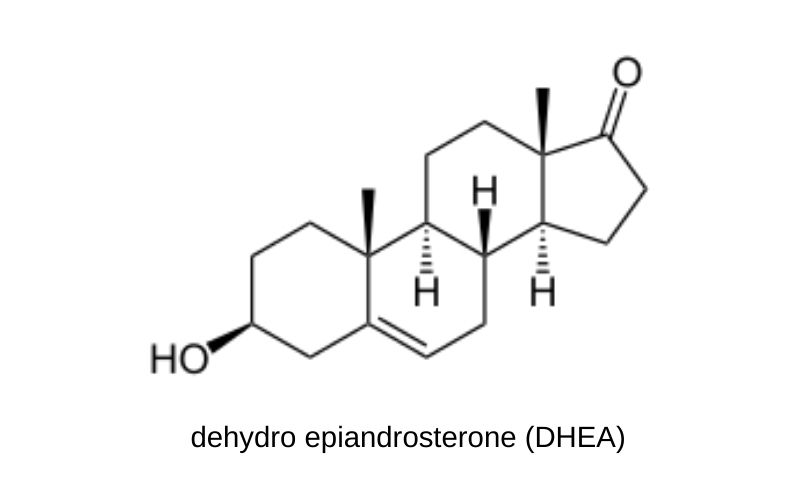
In the hair follicle, the principal pathways involved in conversion of weak androgens like DHEA to more potent androgens are through activity of the enzymes:
- 3 beta – hydroxysteroid dehydrogenase (3 beta – HSD)
- 17 beta – hydroxysteroid dehydrogenase (17 beta – HSD)
In most target organs testosterone can be further metabolized to DHT via the action of 5 -reductase.
Once formed, potent androgens, such as testosterone and DHT, can be removed by conversion back to the weaker 17-ketosteroids, or are metabolized via other enzymatic pathways, including aromatase, which converts androgens to estrogens, and 3 -hydroxysteroid dehydrogenase to form androsterone and androstanediol. The latter can be glucuronidated to form androgen conjugates that are more rapidly cleared from the circulation. Remarkably, some target tissues, such as the hair follicle, show enhanced androgen metabolism and androgen sensitivity.
The enzymes of the 17 beta – hydroxysteroid dehydrogenase (17 beta – HSD) gene family are responsible for a key step in the formation and degradation of androgens and estrogens: catalyzing the interconversion of 17-ketosteroids and their active 17 beta – hydroxysteroid counterparts. The enzymes of the 17 beta – HSD gene family are responsible for the interconversion of DHEA and estradiol, androstenedione and testosterone, estrone and 17 beta –estradiol, as well as of androstenedione and DHT. The reverse oxidative reaction inactivates the potent hydroxysteroids into ketosteroids with little biologic activity. Therefore 17 beta -HSD controls the last step in the formation of all androgens and all estrogens, assuming a key role in the intracellular concentration of all active sex steroids.
17 beta – hydroxysteroid dehydrogenase (17 beta – HSD)
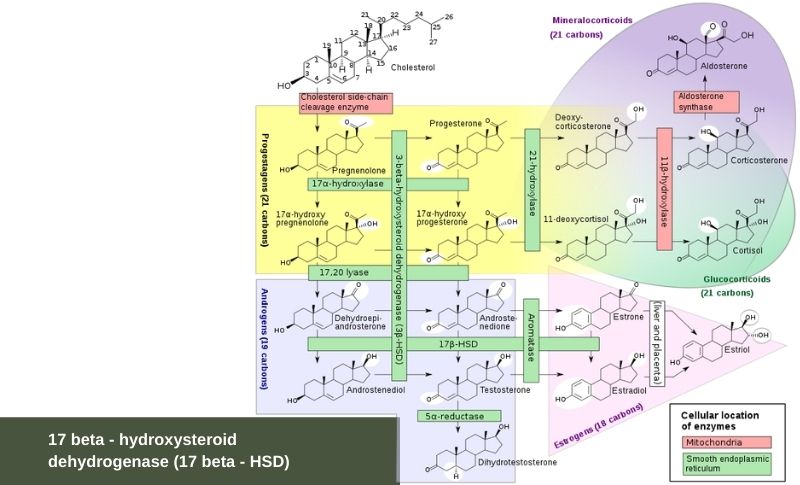
That 17 beta – HSDs are important biologically in testosterone production is corroborated by the fact that deficiency of 17 beta – HSD leads to a form of pseudohermaphroditism. There are at least eight distinct genes described with at least five 17 beta – HSD isoenzymes having individual cell specific expression, substrate specificity, regulation mechanisms, and reductive or oxidative catalytic activity.
- Type I 17 beta – HSD principally controls reduction of estrone to 1713-estradiol, and type II 17 beta- HSD controls the reverse oxidative sequence.
- Types III and V 17 beta – HSD reduce androstenedione to testosterone.
- Types II and IV 17 beta – HSD inactivate testosterone by oxidation to androstenedione.
Histochemically, 17 beta – HSD appears to be located primarily in the outer root sheath of anagen hairs, but appears to diminish in the anagen follicle with progressive baldness. Some studies show a marked decrease in the production of androstenediol from DHEA in the frontal scalp of balding men. Steroid sulfatase, which cleaves DHEA sulfate, the most abundant circulating steroid, to DHEA, has been noted in the dermal papillae of the occipital scalp hair follicle.
Metabolism of the weak androgen DHEA may play an important role in control of androgenetic alopecia. Some target tissues show enhanced androgen metabolism and androgen sensitivity. Circulating DHEA-S may be more rapidly metabolized to DHEA via steroid sulphatase. If increased 3 beta – HSD activity is present, DHEA may be more rapidly converted to androstenedione. Similarly, Androstenedione may be converted to testosterone if 17 beta – HSD activity is present. If target cells convert weak androgens at an accelerated pace, then there will be enhanced conversion of testosterone to DHT. Another reason for increased sensitivity of a target to androgens is believed to involve an increase in the number of androgen receptors.
3 beta – hydroxysteroid dehydrogenase (3 beta – HSD)
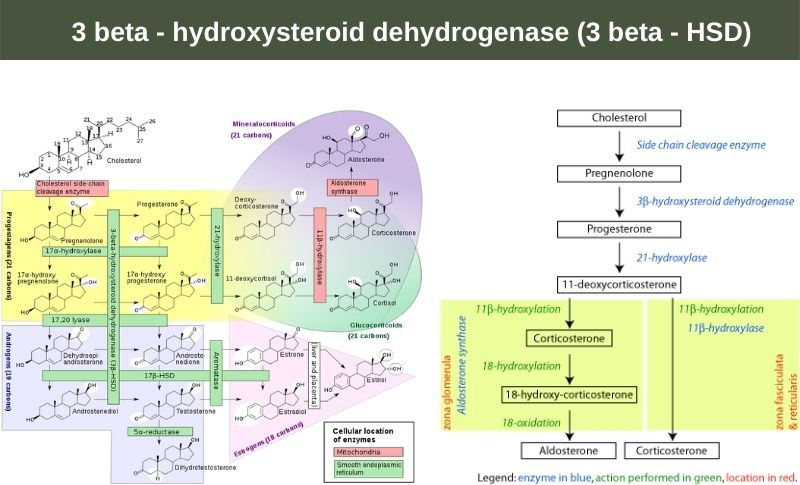
3 beta – HSD catalyzes an essential step in the biosynthesis of all steroid hormones including mineralocorticoids (steroid hormones that are secreted by the adrenal cortex and regulate the balance of water and electrolytes in the body), glucocorticoids (A group of anti-inflammatory steroidlike compounds that are produced by the adrenal cortex, and are sex steroids). Two forms of the enzyme have been described in humans: type I has been recorded primarily in the placenta, skin, and breast and type II in the adrenals and gonads. 3 beta – HSD deficiency has been noted in 17 percent of women with signs of androgen excess. The sebaceous glands in balding skin have been shown to express increased 3 beta – HSD activity when compared to non-balding scalp areas.
3 alpha – HSDs work along with the 5 alpha /5 beta – reductases to convert steroid hormones into tetrahydrosteroids. These oxidoreductase transformations are important in the inactivation of androgens, progestins, and glucocorticoids in the liver and in the regulation of the amount of a given hormone that can bind to a steroid hormone receptor. Thus, NADH and/or NADPH-dependent 35alpha – HSD or 3 beta – HSD can regulate the amount of DHT that can bind to the androgen receptor by controlling interconversion to weak androgens. (Nicotinamide adenine dinucleotide (NAD) and nicotinamide adenine dinucleotide phosphate (NADP) are two important coenzymes found in cells. NADH is the reduced form and NAD+ is the oxidized form of NAD).




